Water Liquid-Vapor Pressure vs Density simulated by GCMC
- FarawayK
-
 Topic Author
Topic Author
- Offline
- New Member
-

Less
More
- Posts: 6
- Thank you received: 1
5 years 6 months ago #532
by FarawayK
Dear Supporters
I'm conducting GCMC simulation about bulk water liquid and vapor under 300K, but the pressure vs density relation is weird
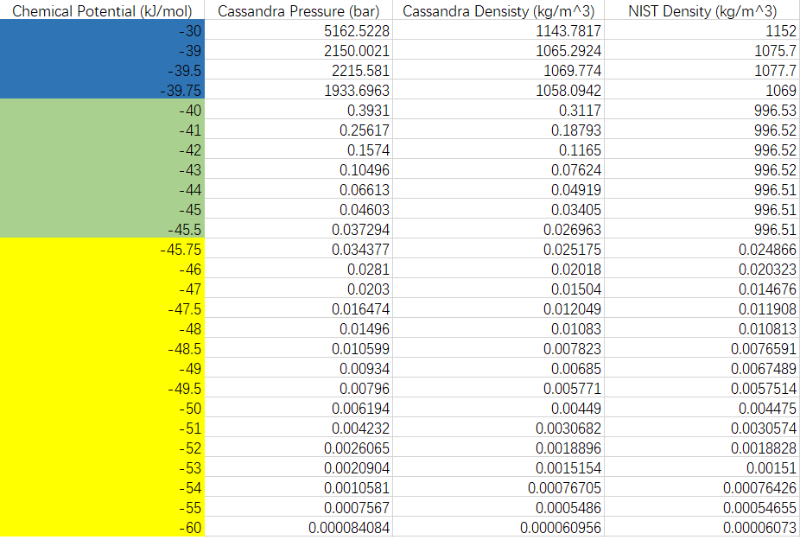
the first row is chemical potential input in the .inp file, the second and third row is pressure and density of water (liquid or vapor) from the simulation results, and the last row is the water density under same pressure from NIST database.
The first question is about the weird density. In the yellow zone, the water is in vapor state, and blue zone is liquid, these are normal. But in the green zone, we could see that the water pressure is higher than water saturation pressure (3.4kPa), or even higher than the saturation pressure of water SPC model (4.4kPa), but the water density is much lower than liquid.
The second question is about the pressure results from simulation, we could see it from the first and second row, when the chemical potential is a little larger than -40kJ/mol, such as -39.75kJ/mol, the water pressure increase sharply to 1933.7bar, which is too high for my target case (1bar~300bar), this question is about how could it be controlled to conduct simulation under lower pressure using GCMC.
water model is spc model, the input .inp file and .mcf file is attached
Kecheng
I'm conducting GCMC simulation about bulk water liquid and vapor under 300K, but the pressure vs density relation is weird
the first row is chemical potential input in the .inp file, the second and third row is pressure and density of water (liquid or vapor) from the simulation results, and the last row is the water density under same pressure from NIST database.
The first question is about the weird density. In the yellow zone, the water is in vapor state, and blue zone is liquid, these are normal. But in the green zone, we could see that the water pressure is higher than water saturation pressure (3.4kPa), or even higher than the saturation pressure of water SPC model (4.4kPa), but the water density is much lower than liquid.
The second question is about the pressure results from simulation, we could see it from the first and second row, when the chemical potential is a little larger than -40kJ/mol, such as -39.75kJ/mol, the water pressure increase sharply to 1933.7bar, which is too high for my target case (1bar~300bar), this question is about how could it be controlled to conduct simulation under lower pressure using GCMC.
water model is spc model, the input .inp file and .mcf file is attached
Kecheng
The following user(s) said Thank You: bassel
Please Log in to join the conversation.
- ryangmullen
-

- Offline
- Administrator
-

Less
More
- Posts: 124
- Karma: 4
- Thank you received: 24
5 years 6 months ago #533
by ryangmullen
Hi Kecheng,
It appears that at the conditions you highlighted green, your simulations are not long enough to converge to the thermodynamically stable density. To review what we should expect from a GCMC simulation:
(*) there is a value of the chemical potential, mu.equil, for which a liquid phase will be in equilibrium with a vapor phase
(*) for mu > mu.equil, the stable phase will be a liquid
(*) for mu < mu.equil, the stable phase will be a vapor
However, the length of a simulation required to converge, depends on the initial configuration. For simulations started from the vapor phase and:
(*) with mu < mu.equil, the simulation will converge quickly. These are the cases you highlighted yellow.
(*) with mu >> mu.equil, the driving force delta.mu = mu - mu.equil is large enough that the simulation length needed to converge isn't too long. These are the cases you highlighted blue.
(*) as mu ---> mu.equil from above, the simulations need to be increasingly longer in order to converge. These are the cases you highlighted green.
For this last case, I'd recommend starting from a relaxed liquid configuration rather than using make_config to generate a vapor configuration. Also, here's a few points to keep in mind:
(*) for a rigid water model, there's no need to use regrowth moves
(*) for a GCMC simulation, outputting the volume is pointless
(*) for a liquid simulation, the pressures fluctuates wildly (+/- 1000 bar) so you will need long simulations if you want to converge <P> to ~1 bar
It appears that at the conditions you highlighted green, your simulations are not long enough to converge to the thermodynamically stable density. To review what we should expect from a GCMC simulation:
(*) there is a value of the chemical potential, mu.equil, for which a liquid phase will be in equilibrium with a vapor phase
(*) for mu > mu.equil, the stable phase will be a liquid
(*) for mu < mu.equil, the stable phase will be a vapor
However, the length of a simulation required to converge, depends on the initial configuration. For simulations started from the vapor phase and:
(*) with mu < mu.equil, the simulation will converge quickly. These are the cases you highlighted yellow.
(*) with mu >> mu.equil, the driving force delta.mu = mu - mu.equil is large enough that the simulation length needed to converge isn't too long. These are the cases you highlighted blue.
(*) as mu ---> mu.equil from above, the simulations need to be increasingly longer in order to converge. These are the cases you highlighted green.
For this last case, I'd recommend starting from a relaxed liquid configuration rather than using make_config to generate a vapor configuration. Also, here's a few points to keep in mind:
(*) for a rigid water model, there's no need to use regrowth moves
(*) for a GCMC simulation, outputting the volume is pointless
(*) for a liquid simulation, the pressures fluctuates wildly (+/- 1000 bar) so you will need long simulations if you want to converge <P> to ~1 bar
Please Log in to join the conversation.
- emarin
-

- Offline
- Administrator
-

Less
More
- Posts: 42
- Karma: 2
- Thank you received: 7
5 years 6 months ago #534
by emarin
Hello!
Could you please confirm which water model reference results are you using from NIST? I'm familiar with the reference simulation website ( www.nist.gov/programs-projects/nist-stan...e-simulation-website ) which I believe doesn't include SPC but includes SPC/E. I think the input files you sent are for the SPC water model.
Thanks!
Could you please confirm which water model reference results are you using from NIST? I'm familiar with the reference simulation website ( www.nist.gov/programs-projects/nist-stan...e-simulation-website ) which I believe doesn't include SPC but includes SPC/E. I think the input files you sent are for the SPC water model.
Thanks!
The following user(s) said Thank You: FarawayK
Please Log in to join the conversation.
- FarawayK
-
 Topic Author
Topic Author
- Offline
- New Member
-

Less
More
- Posts: 6
- Thank you received: 1
5 years 6 months ago #535
by FarawayK
Ryan, thanks for your kindly reply, i tried to conduct simulation from liquid state as initial configuration in the last days
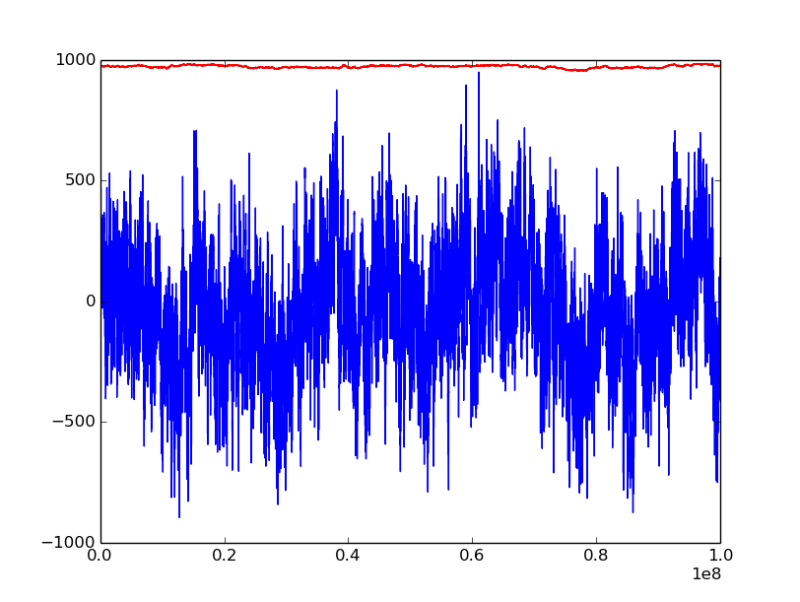
chemical potential mu is -45.5 kJ/mol, initial configuration is liquid state
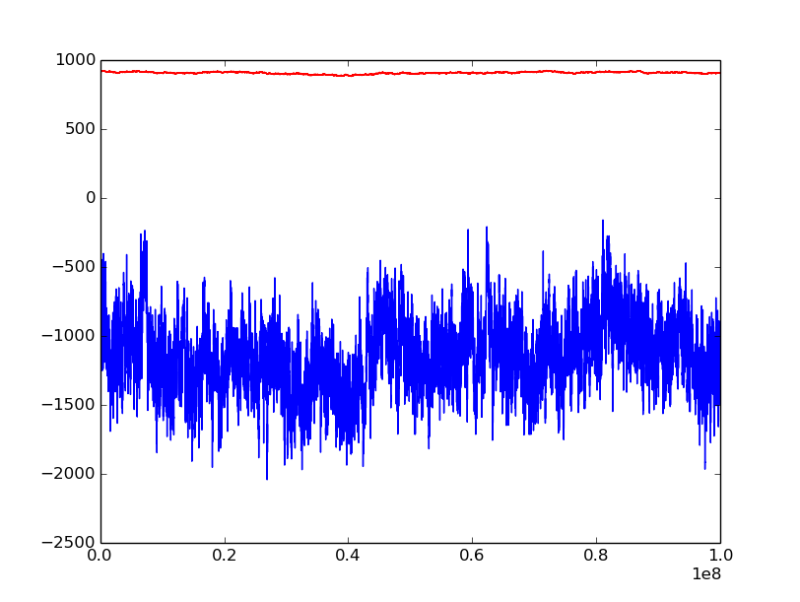
chemical potential mu is -47.5 kJ/mol, initial configuration is liquid state
the horizontal axis is MC step, bule line represents pressure [bar], red line is density of water [kg/m^3]
from the figure we could see that the pressure fluctuation seems very large, the total MC step reaches 1e08, is it still too short to reach euqilibrium?
the other question is about the #Run_Type, i use 'equilibration' to conduct 'unequilibrium system', would 'production' be better to conduct this simulation? would this be the key point?
chemical potential mu is -45.5 kJ/mol, initial configuration is liquid state
chemical potential mu is -47.5 kJ/mol, initial configuration is liquid state
the horizontal axis is MC step, bule line represents pressure [bar], red line is density of water [kg/m^3]
from the figure we could see that the pressure fluctuation seems very large, the total MC step reaches 1e08, is it still too short to reach euqilibrium?
the other question is about the #Run_Type, i use 'equilibration' to conduct 'unequilibrium system', would 'production' be better to conduct this simulation? would this be the key point?
Please Log in to join the conversation.
- ryangmullen
-

- Offline
- Administrator
-

Less
More
- Posts: 124
- Karma: 4
- Thank you received: 24
5 years 6 months ago #536
by ryangmullen
Hi Kecheng,
"the pressure fluctuation seems very large, the total MC step reaches 1e08, is it still too short to reach euqilibrium?"
Pressure fluctuations in a liquid are going to be large, and they won't decrease with a longer simulation. There's nothing you can do to change that. Fluctuations in the average pressure, however, will decrease with simulation length (i.e., as you average more data points, the average will converge to a constant).
I'd think that the energy U, number of molecules N (or density), and U - mu * N would all be better quantities to indicate whether your simulation has converged. Can you also share what your insertion and deletion acceptance ratios are? I'd think you'd want to see several thousand insertions and several thousand deletions to indicate that you're getting good sampling.
"would 'production' be better to conduct this simulation?"
The only difference between equilibration and production Run_Types is that, in equilibration, Cassandra will change the maximum distance and maximum angle used for translation and rotation moves until the acceptance ratio is 50%. From the input file you uploaded earlier, it looks like these values start at 2.0 Angstroms and 180 degrees. In my experience, these are too large for liquid water. Cassandra will change these values every 600 moves (based on your input file) until they reach something like 1.0 Angstrom and 20 degrees (=0.35 radians). Each change will be written to the log file.
In a production run, the max_width and max_angle will not change. They will stay at whatever value is in your input file, or if you start from a checkpoint file, to whatever values are in your checkpoint file.
Ryan
"the pressure fluctuation seems very large, the total MC step reaches 1e08, is it still too short to reach euqilibrium?"
Pressure fluctuations in a liquid are going to be large, and they won't decrease with a longer simulation. There's nothing you can do to change that. Fluctuations in the average pressure, however, will decrease with simulation length (i.e., as you average more data points, the average will converge to a constant).
I'd think that the energy U, number of molecules N (or density), and U - mu * N would all be better quantities to indicate whether your simulation has converged. Can you also share what your insertion and deletion acceptance ratios are? I'd think you'd want to see several thousand insertions and several thousand deletions to indicate that you're getting good sampling.
"would 'production' be better to conduct this simulation?"
The only difference between equilibration and production Run_Types is that, in equilibration, Cassandra will change the maximum distance and maximum angle used for translation and rotation moves until the acceptance ratio is 50%. From the input file you uploaded earlier, it looks like these values start at 2.0 Angstroms and 180 degrees. In my experience, these are too large for liquid water. Cassandra will change these values every 600 moves (based on your input file) until they reach something like 1.0 Angstrom and 20 degrees (=0.35 radians). Each change will be written to the log file.
In a production run, the max_width and max_angle will not change. They will stay at whatever value is in your input file, or if you start from a checkpoint file, to whatever values are in your checkpoint file.
Ryan
Please Log in to join the conversation.
- ryangmullen
-

- Offline
- Administrator
-

Less
More
- Posts: 124
- Karma: 4
- Thank you received: 24
5 years 6 months ago - 5 years 6 months ago #537
by ryangmullen
Also, from your plots it appears that
(*) at mu = -45.5 kJ/mol, <P> ~= 0-100 bar
(*) at mu = -47.5 kJ/mol, <P> ~= -1100 bar
The negative pressure indicates that the liquid is under tension (i.e., the system wants to be a vapor at this mu). This is consistent with your previous result that <P> = 0.02 bar at mu = -47.5 kJ/mol. Similar to the problems you had converging to a liquid when you started from a vapor for -45.5 < mu < -40 kJ/mol, you will have slow convergence to the vapor state if you start from a liquid for mu < -45.5 kJ/mol.
Ryan
(*) at mu = -45.5 kJ/mol, <P> ~= 0-100 bar
(*) at mu = -47.5 kJ/mol, <P> ~= -1100 bar
The negative pressure indicates that the liquid is under tension (i.e., the system wants to be a vapor at this mu). This is consistent with your previous result that <P> = 0.02 bar at mu = -47.5 kJ/mol. Similar to the problems you had converging to a liquid when you started from a vapor for -45.5 < mu < -40 kJ/mol, you will have slow convergence to the vapor state if you start from a liquid for mu < -45.5 kJ/mol.
Ryan
Last Edit: 5 years 6 months ago by ryangmullen.
Please Log in to join the conversation.
Time to create page: 0.275 seconds